Update on the UK approval of gene therapy for MLD
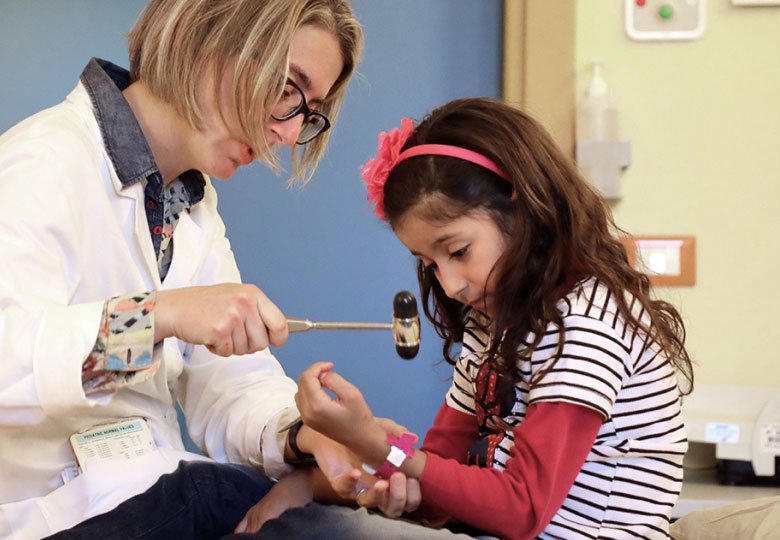
Posted on: 18/12/2020ArchAngel MLD Trust and Ava's mother Georgina were nominated as 'expert witnesses' and both have just submitted extensive evidence to The National Institute of Health & Care Excellence (NICE) in support of an application to have Gene Therapy for MLD approved for use by NHS England. If approved, this remarkable treatment could offer UK children with a timely diagnosis the same second chance of life which it has (via clinical trial) has afforded Ava. Having personal and professional experience of meeting many MLD affected families - both treated and untreated - we have great confidence in the efficacy of this treatment and will do our utmost to ensure that we unequivocally communciate the brutality of this disease Vs the salvation of gene therapy at the Committee hearings (next spring). It is a complex process and timescales are not confirmed, however it is possible that NICE may reach a decision by summer 2021. The approval would not only offer a literal life-line to newly diagnosed patients whom are eligible, but also provide us with a vital step towards being to apply to have MLD added to the UK newborn heelprick test. Without early intervention, sadly most children are diagnosed too late to be able to benefit from this ground-breaking medicine. Please see our NBS Campaign page for further information. The picture shows one of our heroes, the wonderful Dr. Francesca Fumagalli, a Principal Neurologist at the Telethon Institute of Gene Therapy in Milan.
©2024 Copyright ArchAngel MLD Trust
Website by Modern Websites