Run for ArchAngel!
Mon Mar 4 2024
We have now partnered with Real Buzz to secure charity places on world-wide marathons and half marathons! Full support is given to runners in their fundrasing efforts. More information can be found at their website [link]
Rare Disease Day
Tue Feb 28 2023
Today is Rare Disease Day. Thinking of our MLD families, who struggle and grieve today and every day. We see you.
Shaw family press coverage
Wed Feb 15 2023
Some excellent coverage in today's press of the first MLD affected child to receive Libmeldy in the UK. Thanks to the incredibly strong Shaw family for sharing their story and raising important awareness of MLD and the need for Newborn Screening.
First UK child to receive Libmeldy
Tue Feb 14 2023
The BBC have made an excellent documentary following Teddi Shaw, the first UK patient to receive transformative gene therapy for MLD, 'Libmeldy'. This is the therapy which we, and many dedicated others, fought so hard to get approved in the UK. Hats off to all involved, especially the remarkable teams at Manchester Children's Hospital and Ospedale San Raffaele in Milan and the extra....
Today is International Neonatal Screening Day
Tue Jun 28 2022
Today is INTERNATIONAL NEONATAL SCREENING TODAY. Newborn screening has the power to save or dramatically improve the lives of those with rare diseases, by identifying conditions and allowing for treatment at the earliest opportunity. ArchAngel is proud to be a member of the UK Newborn Screening Collaborative, comprising 14 key rare disease patient organisations, plus Genetic Alliance UK. Collectiv....
Update on newborn screening for MLD
Sat May 7 2022
The UK NSC Secretariat have confirmed that they will commission an ‘evidence map’ to further examine the case for MLD. We expect a further update from the NSC in 2023. Whilst this is a very positive step, unfortunately the assessment of any NBS application in the UK is a very lengthy and challenging process due to outdated bureaucracy (see NBS page). We hope to continue to work with th....
Good News for NBS on Rare Disease Day
Mon Feb 28 2022
Today is Rare Disease Day and we are thinking of all the families living with rare disease every single day, especially our MLD warriors.
We are also delighted to share the England Rare Disease Action Plan which was published by the Department of Health & Social Care today. It contains an important committment to improving newborn screening, in line with the key policy chang....
We are also delighted to share the England Rare Disease Action Plan which was published by the Department of Health & Social Care today. It contains an important committment to improving newborn screening, in line with the key policy chang....
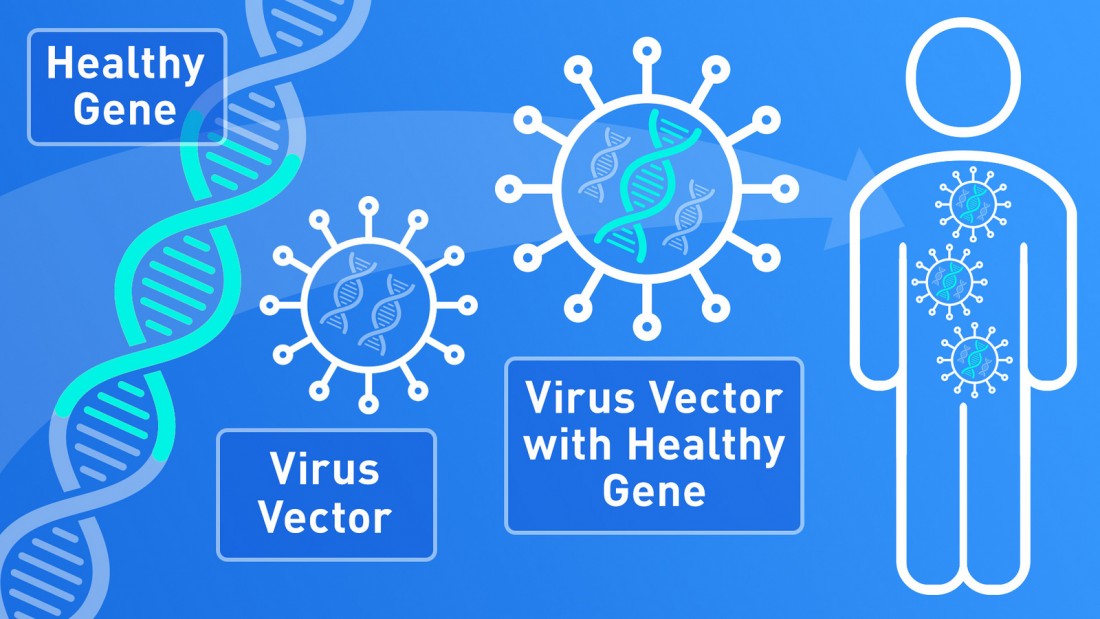
Gene therapy APPROVED for NHS use
Fri Feb 4 2022
The life-changing gene therapy which Ava received in Milan in 2014 has been APPROVED for use by the NHS! Today's announcement on the approval of Libmeldy for use by NHS England is a momentous one for future MLD affected families, as well as gene therapies for other conditions. We are beyond grateful to all of the clinicians, scientists and patient advocates who have helped made....
IMPORTANT NBS UPDATE
Mon Dec 6 2021
Very happy to report that a formal application to add Metachromatic Leukodystrophy to the UK Newborn Bloodspot (NBS) programme has now been made to the National Screening Committee (NSC). This is an important milestone for the MLD community and one which could not have been possible without the important support and ongoing collaboration of many brilliant scientists and heroic clinicians.
Unfort....
UPDATE ON UK APPROVAL OF GENE THERAPY
Sat Jul 10 2021
NICE unfortunately leans towards a ‘NO’ for the treatment of Metachromatic Leukodystrophy (MLD).
NICE (National Institute of Clinical Excellence) the drug decision making body have publicly released their interim decision today (9 July 2021) not to recommend OTL-200 (Libmeldy), the treatment for individuals suffering from both, late infantile and early juvenile Metachromatic Leuko....
NICE (National Institute of Clinical Excellence) the drug decision making body have publicly released their interim decision today (9 July 2021) not to recommend OTL-200 (Libmeldy), the treatment for individuals suffering from both, late infantile and early juvenile Metachromatic Leuko....
©2024 Copyright ArchAngel MLD Trust
Website by Modern Websites